2018年
原著論文
Use of modified U1 small nuclear RNA for rescue from exon 7 skipping caused by 5-splice site mutation of human cathepsin A gene
Naoshi Yamazaki, Keisuke Kanazawa, Maria Kimura, Hironobu Ike, Makiko Shinomiya,
Shouko Tanaka, Yasuo Shinohara, Noriaki Minakawa, Kohji Itou, Yoshiharu Takiguchi. Gene. 2018, 677, 41–48. [doi.org/10.1016/j.gene.2018.07.030]
Glycosylation reactions mediated by hypervalent iodine: application to the synthesis of nucleosides and carbohydrates
Yuichi Yoshimura, Hideaki Wakamatsu, Yoshihiro Natori, Yukako Saito and Noriaki MinakawaBeilstein J. Org. Chem. 2018, 14, 1595–1618 [doi:10.3762/bjoc.14.137]
Ribavirin-related compounds exert in vitroinhibitory effects toward rabies virus
Paulina D. Anindita, Michihito Sasaki, Kazuma Okada, Naoto Ito, Makoto Sugiyama,
Noriko Saito-Tarashima, Noriaki Minakawa,Satoshi Shuto, Satoko Otsuguro, Satoshi Ichikawa,
Akira Matsuda, Katsumi Maenaka, YasukoOrba, HirofumiSawa
Antiviral Res. 2018, 154, 1–9 [10.1016/j.antiviral.2018.03.011]
総説・著書
Four-hydrogen-bonding base pairs in oligonucleotides: design, synthesis and properties, Synthesis of Therapeutic Oligonucleotides
Noriko Saito-Tarashima, Akira Matsuda, Noriaki Minakawa.
Springer, in press. 2018, 147–169 [doi.org/10.1007/978-981-13-1912-9_9]
RNA bioisoster: Chemistry and properties of 4’-thioRNA and 4’-selenoRNA, Synthesis of Therapeutic Oligonucleotides
Noriaki Minakawa, Akira Matsuda, Noriko Saito-Tarashima
Springer, in press. 2018, 233–252 [doi.org/10.1007/978-981-13-1912-9_14]
「第16章 化学修飾DNAを利用したRNAi創薬」
田良島典子、南川典昭
『CSJ Current Review 生命機能に迫る分子化学』化学同人、2018, 144–150.
Unnatural Base Pairs for Synthetic Biology
Noriko Saito-Tarashima, Noriaki Minakawa
Chem. Pharm. Bull. 2018, 66, 132–138 [DOI: 10.1248/cpb.c17-00685]
2017年
原著論文
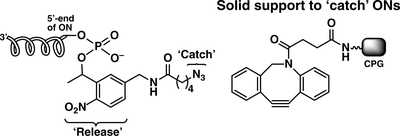
A ‘catch and release’ strategy towards HPLC-free purification of synthetic oligonucleotides by a combination of the strain-promoted alkyne-azide cycloaddition and the photocleavage
Yosuke Igata, NorikoSaito-Tarashima, Daiki Matsumoto, Kazuyuki Sagara, Noriaki Minakawa
Bioorg. Med Chem. 2017, 25, 5962–5967 [DOI: 10.1016/j.bmc.2017.09.014]
Synthesis of 4′-Selenoribonucleosides, the Building Blocks of 4′-SelenoRNA, Using a Hypervalent Iodine
Noriko Saito-Tarashima, Masashi Ota,Noriaki Minakawa
Curr.Proc. Nucl.Acids., 2017, 70, 1.40.1–1.40.21 [DOI: 10.1002/cpnc.34]
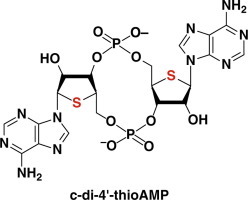
Synthesis and evaluation of c-di-4’-thioAMP as an artificial ligand for c-di-AMP riboswitch
Kazuto Shiraishi,Noriko Saito-Tarashima,Yosuke Igata,Keiji Murakami,Yasuko Okamoto,Yoichiro Miyake,Kazuhiro Furukawa,Noriaki Minakawa
Bioorg. Med. Chem., 2017, 25, 3883–3889 [DOI: 10.1016/j.bmc.2017.05.042]
The AMPK/mTOR pathway is involved in D-dopachrome tautomerase gene transcription in adipocytes differentiated from SGBS cells, a human preadipocyte cell line
Takeo Iwata, Kyoko Kuribayashi, Masahiko Nakasono, Noriko Saito-Tarashima, Noriaki Minakawa, Noriko Mizusawa, Rie Kido, Katsuhiko Yoshimoto
Cytokine, 2017, 96, 195–202 [DOI: 10.1016/j.cyto.2017.04.017]
2016年
原著論文
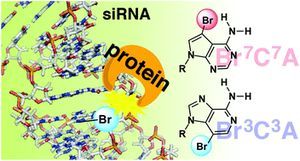
Groove modification of siRNA duplexes to elucidate siRNA–protein interactions using 7-bromo-7-deazaadenosine and 3-bromo-3-deazaadenosine as chemical probes
Noriko Saito-Tarashima, Hirotaka Kira, Tomoya Wada, Kazuya Miki, Shiho Ide, Naoshi Yamazaki, Akira Matsuda and Noriaki Minakawa
Org. Biomol. Chem., 2016,14, 11096-11105 [DOI:10.1039/C6OB01866A]
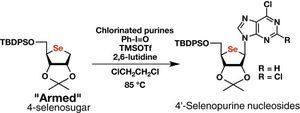
Practical synthesis of 4′-selenopurine nucleosides by combining chlorinated purines and ‘armed’ 4-selenosugar
Kazuki Ishii, Noriko Saito-Tarashima, Masashi Ota, Seigi Yamamoto, Yasuko Okamoto, Yoshiyuki Tanaka and Noriaki Minakawa
Tetrahedron, 2016, 72, 6589-6594 [DOI:10.1016/j.tet.2016.08.071]
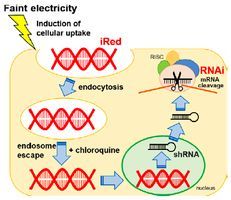
The novel functional nucleic acid iRed effectively regulates target genes following cytoplasmic delivery by faint electric treatment
Mahadi Hasan, Noriko Tarashima, Koki Fujikawa, Takashi Ohgita, Susumu Hama, Tamotsu Tanaka, Hiroyuki Saito, Noriaki Minakawa and Kentaro Kogure
Sci. Tech. Adv. Mater., 2016, 17, 554-562 [DOI:10.1080/14686996.2016.1221726]
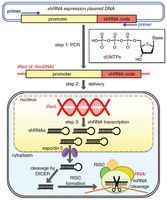
Gene Silencing Using 4′-thioDNA as an Artificial Template to Synthesize Short Hairpin RNA Without Inducing a Detectable Innate Immune Response
Noriko Tarashima, Hidenori Ando, Takamitsu Kojima, Nozomi Kinjo, Yosuke Hashimoto, Kazuhiro Furukawa, Tatsuhiro Ishida and Noriaki Minakawa
Mol. Ther. Nucleic Acids, 2016, 5, e274 [DOI:10.1038/mtna.2015.48]
2016_Sci. Technol. Adv. Mater._1_result. Gene Silencing Using 4′-thioDNA as an Artificial Template to Synthesize Short Hairpin RNA Without Inducing a Detectable Innate Immune Response
Noriko Tarashima, Hidenori Ando, Takamitsu Kojima, Nozomi Kinjo, Yosuke Hashimoto, Kazuhiro Furukawa, Tatsuhiro Ishida and Noriaki Minakawa
Mol. Ther. Nucleic Acids, 2016, 5, e274 [DOI:10.1038/mtna.2015.48]
総説・著書
1. 田良島典子、南川典昭
生物学的等価性を指向した化学修飾DNAによる核酸創薬研究
『核酸医薬の創製と応用展開』(シーエムシー出版) 2016年, pp70-78.
2015年
原著論文
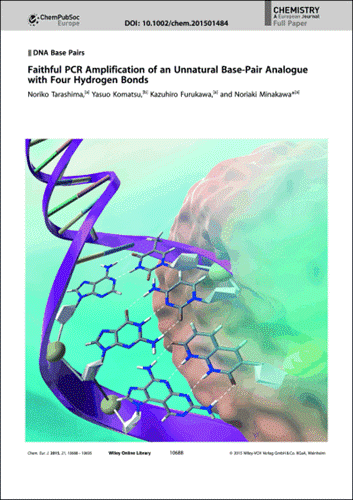
Faithful PCR Amplification of an Unnatural Base-Pair Analogue with Four Hydrogen Bond
Noriko Tarashima,Yasuo Komatsu,Kazuhiro Furukawa,and Noriaki Minakawa
Chem. Eur.J.,2015,21,10688-10695 [DOI: 10.1002/chem.201583062]
Synthesis of DNA fragments containing 2’-deoxy-4’-selenonucleoside units using DNA polymerases:
comparison of dNTPs with O, S and Se at the 4’-position in replication
Noriko Tarashima, Tatsuya Sumitomo, Hidenori Ando, Kazuhiro Furukawa, Tatsuhiro Ishida and Noriaki Minakawa*
Organic & Biomolecular Chemistry.,2015,13,6949-6952 [DOI: 10.1039/c5ob00941c ]
Transcription of 4′-thioDNA templates to natural RNA in vitro and in mammalian cells
Hideto Maruyama, Kazuhiro Furukawa, Hiroyuki Kamiya, Noriaki Minakawa and Akira Matsuda
Chem. Commun. 2015,51, 7887-7890 [DOI: 10.1039/C4CC08862J]
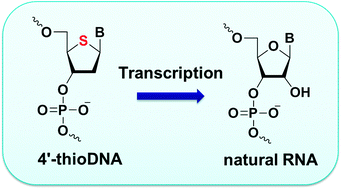
Graphical abstract: Transcription of 4′-thioDNA templates to natural RNA in vitro and in mammalian cells
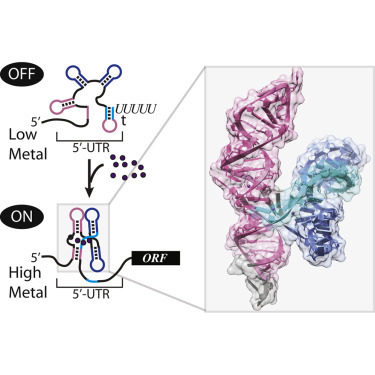
Bacterial riboswitches cooperatively bind Ni2+ or Co2+ ions and control expression of heavy metal transporters
Kazuhiro Furukawa, Arati Ramesh, Zhiyuan Zhou, Zasha Weinberg,Tenaya Vallery, Wade C. Winkler, Ronald R. Breaker
Mol. Cell.19, 2015, 57, 1088–1098 [DOI: 10.1016/j.molcel.2015.02.009]
2014年
原著論文
Chemistry, properties, and in vitro and in vivo applications of 2′-O-methoxyethyl-4′-thioRNA, a novel hybrid type of chemically modified RNA
Yota Saito,Yosuke Hashimoto, Mai Arai, Noriko Tarashima, Tadashi Miyazawa, Kazuya Miki, Mayumi Takahashi, Kazuhiro Furukawa, Naoshi Yamazaki, Akira Matsuda,Tatsuhiro Ishida, Noriaki Minakawa
ChemBioChem, 2014,15, 2535–2540 [DOI: 10.1002/cbic.201402398]
Link to full-size graphical abstract
First synthesis of fully modified 4'-selenoRNA and 2'-OMe-4'-selenoRNA based on the mechanistic considerations of an unexpected strand break
Noriko Tarashima, Koya Hayashi, Maki Terasaki, Hirotsugu Taniike, Yusuke Inagaki, Kenji Hirose, Kazuhiro Furukawa, Akira Matsuda, Noriaki Minakawa
Org. Lett. 2014,16, 4710–4713 [DOI: 10.1021/ol502077h]
Abstract Image
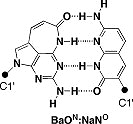
Development of a new dumbbell-shaped decoy DNA using a combination of the unnatural base pair ImON:NaNO and a CuAAC reaction
Yosuke Higuchi, Kazuhiro Furukawa, Tadashi Miyazawa, Noriaki Minakawa
Bioconjugate Chem. 2014, 25, 1360–1369 [DOI: 10.1021/bc500225r]
Abstract Image
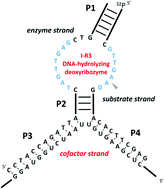
Allosteric control of a DNA-hydrolyzing deozyribozyme with short oligonucleotides and its application in DNA logic gates
Kazuhiro Furukawa, Noriaki Minakawa
Org. Biomol. Chem. 2014, 12, 3344–3348 [DOI: 10.1039/C4OB00451E]
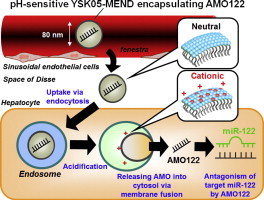
5. The systemic administration of an anti-miRNA oligonucleotide encapsulated pH-sensitive liposome results in reduced level of hepatic microRNA-122 in mice
Hiroto Hatakeyama, Manami Murata, Yusuke Sato, Mayumi Takahashi, Noriaki Minakawa, Akira Matsuda, Hideyoshi Harashima
Jounal of Controlled Release, 2014, 173, 43-50
総説・著書
1. Minakawa N, Matsuda A
Design,Characterization,and Application of Imidazopyridopyrimidine:Naphthyridine Base-Pairing Motifs Consisting of Four Hydroren Bonds
Chemical Biology of Nucleic Acids:Volker.A Erdmann,Wojciech T.Markiewicz,Jan Barciszewski,Eds:Springer 2014 p113-129
2 Kazuhiro Furukawa, Hongzhou Gu, Ronald Breaker
In Vitro Selection of allosteric ribozymes that sense the bacterial second messenger c-di-GMP
Methods in Molecular Biology, 2014, 1111, 209-220
2013年
原著論文
1. James W Nelson, Narasimhan Sudarsan, Kazuhiro Furukawa, Zasha Weinberg, Joy X Wang, Ronald R Breaker
Riboswitches in eubacteria sence the second messenger c-di AMP
Nat. Chem. Biol., 2013, 9, 834-839
2. Mayumi Takahashi, Naoki Yamada, Hiroto Hatakeyama, Manami Murata, Yusuke Sato, Noriaki Minakawa, Hideyoshi Harashima, and Akira Matsuda
In vitoro optimization of 2'-OMe-4'-thioribonucleoside-modified anti-microRNA oligonucleotides and its targeting delivery to mouse liver using a liposomal nanoparticle
Nucleic Acids Res. 2013, 1-9
3. Takamitsu Kojima, Kazuhiro Furukawa, Hideto Maruyama, Naonori Inoue, Noriko Tarashima, Akira Matsuda, Noriaki Minakawa
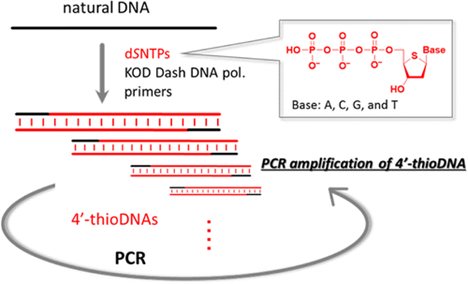
PCR Amplification of 4'-ThioDNA Using 2'-Deoxy-4'-thionucleoside 5'-triphosphates
ACS Synth. Biol. 2013, 2 (9), 529–536
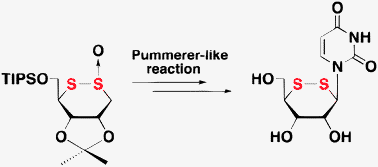
4. Tadashi Miyazawa, Kouhei Umezaki, Noriko Tarashima, Kazuhiro Furukawa, Takashi Ooi and Noriaki Minakawa
Synthesis of a novel 1,2-dithianenucleoside via Pummerer-like reaction, followed by Vorbruggen glycosylation between a 1,2-dithiane derivative and uracil
Chem. Commun. 2013, 49, 7851-7853
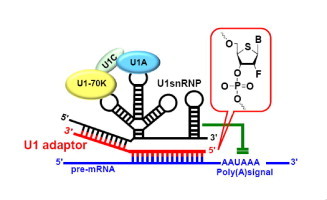
5. Yusaku Kikuchi, Naoshi Yamazaki, Noriko Tarashima, Kazuhiro Furukawa, Yoshiharu Takiguchi, Kohji Itoh, and Noriaki Minakawa
Gene suppression via U1 small nuclear RNA interference (U1i) machinery using oligonucleotides containing 2'-modified-4'-thionucleosides
Bioorg. Med. Chem. 2013, 21, 5292-5296
6. Hongzhou Gu, Kazuhiro Furukawa, Zasha Weinberg, Daniel Berenson, Ronald Breaker
Small, Highly Active DNAs That Hydrolyze DNA
J. Am. Chem. Soc., 2013, 135 (24) 9121-9129
Abstract Image
総説・著書
1. 古川和寛
病原性細菌の抗生物質耐性を司るRNAスイッチ
ファルマシア, 2013, 49, 905
2. Minakawa N
Development of RNA Medicine Using 4'-thioDNA.
Yakugaku Zasshi. 2013, 133, 53-60. (in Japanese)
3. 野村勇作、柏木怜、佐藤浩輔、南川典昭、松田彰
4本の水素結合対を持つ核酸塩基の設計(2)-DNA,RNAポリメラーゼによる認識-
Antisense 2013, 17 , 2, 3-13
2012年
原著論文
1. Tarashima N, Higuchi Y, Komatsu Y, Minakawa N.
A practical post-modification synthesis of oligodeoxynucleotides containing 4,7-diaminoimidazo[5',4':4,5]pyrido[2,3-d]pyrimidine nucleoside.
Bioorg Med Chem. 2012, 20, 7095-7100
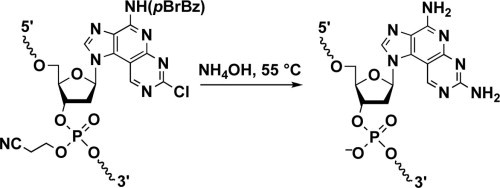
2. Takahashi M, Nagai C, Hatakeyama H, Minakawa N, Harashima H, Matsuda A.
Intracellular stability of 2'-OMe-4'-thioribonucleoside modified siRNA leads to long-term RNAi effect.
Nucleic Acids Res. 2012, 40, 5787-5793
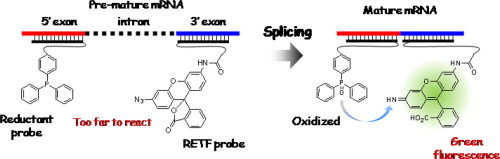
3. Tamura Y, Furukawa K, Yoshimoto R, Kawai Y, Yoshida M, Tsuneda S, Ito Y, Abe H.
Detection of pre-mRNA splicing in vitro by an RNA-templated fluorogenic reaction.
Bioorg Med Chem Lett. 2012, 22,7248-7251
4. Furukawa K, Gu H, Sudarsan N, Hayakawa Y, Hyodo M, Breaker RR.
Identification of ligand analogues that control c-di-GMP riboswitches.
ACS Chem. Biol. 2012, 7, 1436-1443
Abstract Image
5. Gu H, Furukawa K, Breaker RR.
Engineered allosteric ribozymes that sense the bacterial second messenger cyclic diguanosyl 5'-monophosphate.
Anal. Chem. 2012, 84, 4935-4941
Abstract Image
2011年
原著論文
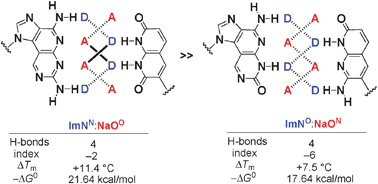
1. K. Kuramoto, N. Tarashima, Y. Hirama, Y. Kikuchi, N. Minakawa, and A. Matsuda
New imidazopyridopyrimidine:naphthyridine base-pairing motif, ImNN:NaOO, consisting of a DAAD:ADDA hydrogen bonding pattern, markedly stabilize DNA duplexes
Chem. Commun. 2011,47,10818-10820
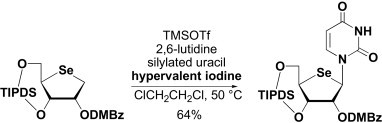
2. H. Taniike, Y. Inagaki, A. Matsuda, and N. Minakawa
Practical synthesis of 4′-selenopyrimidine nucleosides using hypervalent iodine
Tetrahedron. 2011,67,7977-7982.
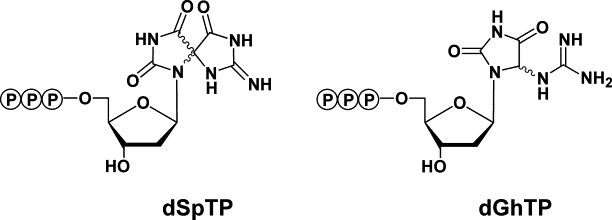
3. M. Hori, T. Suzuki, N. Minakawa, A. Matsuda, H. Harashima, H. Kamiya
Mutagenecity of secondary oxidation products of 8-oxo-7,8-dihydro-2’-deoxyguanosine 5’-triphosphate (8-hydroxy-2’-deoxyguanosine 5’-triphosphate)
Mut. Res. 2011, 714, 11-16.
4. Y. Hirama, N. Minakawa, and A. Matsuda
Synthesis and characterization of oligodeoxynucleotides containing a novel tetraazabenzo[cd]azulene:naphthyridine base pair
Bioorg. Med. Chem. 2011, 19, 352-358.
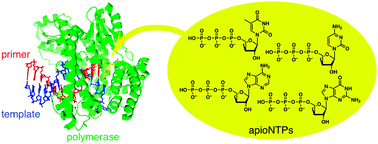
5. M. Kataoka, Y. Kouda, K. Sato,N. Minakawa, and A. Matsuda
Highlhy efficient enzymatic synthesis of 3'-deoxyapionucleic acid (apioNA) having the four natural nucleobases
Chem. Commun. 2011, 47, 8700-8702.
2010年
原著論文
1. K. Tomaya, M. Takahashi, N. Minakawa, and A. Matsuda
A convenient RNA synthesis using a phosphoramidite possessing a biotinylated photocleavable group
Org. Lett. 2010, 12, 3836-3839
Abstract Image
2. Y. Hirama, H. Abe, N. Minakawa, and A. Matsuda
Synthesis and properties of a novel nucleoside derivative possessing a 2,3,5,6-tetraazabenzo[cd]azulene skeleton
Tetrahedron 2010, 66, 8402-8406.
2009年
原著論文
1. N. Minakawa, S. Ogata, M. Takahashi, A. Matsuda
Selective recognition of unnatural imidazopyridopyrimidine:naphthyridine base pairs consisting of four hydrogen bonds by the Klenow fragment
J. Am. Chem. Soc., 2009, 131, 1644-1645.
Abstract Image
2. M. Takahashi, N. Minakawa, A. Matsuda
Synthesis and characterization of 2’-modified-4’-thioRNA: a comprehensive comparison of nuclease stability
Nucleic Acids Res. 2009, 37, 1353-1362.
3. S. Ogata, M. Takahashi, N. Minakawa, A. Matsuda
Unnatural imidazopyridopyrimidine:naphthyridine base pairs: selective incorporation and extension reaction by Deep Vent (exo–) DNA polymerase
Nucleic Acids Res. 2009, 37, 5602-5609.
総説・著書
1. 南川 典昭,松田 彰
siRNA分子のデザインとターゲッティング
『がん分子標的治療研究 実践マニュアル』(金芳堂)2009年,pp164–170.
2008年以前
原著論文
1. N. Minakawa, Y. Kawano, S. Murata, N. Inoue, A. Matsuda
Oligodeoxynucleotides containing 3-bromo-3-deazaadenine and 7-bromo-7-deazaadenine nucleosides as chemical probes to investigate DNA-protein interaction.
ChemBioChem 2008, 9, 464–470.
2. M. Takahashi, S. Daidouji, M. Shiro, N. Minakawa, A. Matsuda
Synthesis and Crystal Structure of 2’-Deoxy-2’-fluoro-4’-thionucleosides: Substrates for the Synthesis on Novel Modified RNAs.
Tetrahedron 2008, 64, 4313-4324.
3. A. Matsugami, T. Ohyama, M. Inada, N. Inoue, N. Minakawa, A. Matsuda, M. Katahira
Unxepected A-form formation of 4’-thioDNA in solution, revealed by NMR, and the implications as to the mechanism of nuclease-resistance.
Nucleic Acids Res. 2008, 36, 1805-1812.
4. N. Minakawa, M. Sanji, Y. Kato, A. Matsuda
Investigations toward the selection of fully-modified 4’-thioDNA aptamers: Optimization of in vitro transcription steps in the presence of 4’-thioNTPs
Bioorg. Med. Chem. 2008, 16, 9450-9456.
5. S. Hoshika, N. Minakawa, A. Shionoya, K. Imada, N. Ogawa, A. Matsuda.
Study of modification pattern–RNAi activity relationships using siRNAs modified with 4’-thioribonucleosides.
ChemBioChem 2007, 8, 2133–2138.
6. N. Inoue, A. Shionoya, N. Minakawa, A. Kawakami, N. Ogawa, A. Matsuda
Amplification of 4’-thioDNA in the presence of 4’-thio-dTTP and 4’-thio-dCTP, and 4’-thioDNA-derected transcription in vitro and in cells.
J. Am. Chem. Soc. 2007, 129, 15424–15425.
7. N. Minakawa, K. Kuramoto, S. Hikishima, A. Matsuda
Four hydrogen-bonding motifs in oligonucleotides
ARKIVOC 2006, 7, 326–337.
8. S. Ichikawa, N. Minakawa, S. Shuto, M. Tanaka, T. Sasaki, A. Matsuda
Synthesis of 3’--carbamoylmethylcytidine (CAMC) and its derivatives as potential antitumor agents
Org. Biomol. Chem. 2006, 4, 1284–1296.
9. N. Inoue, N. Minakawa, A. Matsuda
Synthesis and Properties of 4’-ThioDNA: Unexpected RNA-like Behavior of 4’-ThioDNA
Nucleic Acids Res. 2006, 34, 3476–3483.
10. S. Hikishima, N. Minakawa, K. Kuramoto, S. Ogata, A. Matsuda
Synthesis and Characterization of Oligodeoxynucleotides Containing Naphthyridine:Imidazopyridopyrimidine Base Pairs on their Sticky Ends. Application to Thermally Stabilized Decoy Molecules.
ChemBioChem 2006, 7, 1970–1975.
11. H. Kamiya, C. Cadene-Amaro, L. Dugué, H. Yakushiji, N. Minakawa, A. Matsuda, S. Pochet, Y. Nakabeppu, H. Harashima.
Recognition of Nucleotide Analogs Containing the 7,8-Dihydro-8-oxo Structure by the Human MTH1 Protein.
J. Biochem. 2006, 140, 843–849.
12. S. Hikishima, N. Minakawa, K. Kuramoto, Y. Fujisawa, M. Ogawa, A. Matsuda
Synthesis of 1,8-Naphthyridine C-Nucleosides and Their Base-Pairing Properties in Oligodeoxynucleotides: Thermally Stable Naphthyridine:Imidazopyridopyrimidine Base-Pairing Motifs.
Angew. Chem. Int. Ed. 2005, 44, 596–598.
13. D. Kaga, N. Minakawa, A. Matsuda
Synthesis of 2’-C-Methyl-4’-thiocytidine: Unexpected Anomerization of the 2’-keto-4’-thionucleoside Precursor.
Nucleosides Nucleotides Nucleic Acids 2005, 24, 1789–1800.
14. M. M. Changalov, G. D. Ivanova, M. A. Rangelov, P. Acharya, S. Acharya, N. Minakawa, A. Foldesi, I. B. Stoineva, V. M. Yomtova, C. D. Roussev, A. Matsuda, J. Chattopadhyaya, D. D. Petkov
2’/3’-O-Peptidyl Adenosine as a General Base catalyst of it Own External Peptidyl Transfer: Implications for the Ribosome Catalytic Mechanism.
ChemBioChem 2005, 6, 992–996.
15. S. Hoshika, N. Minakawa, H. Kamiya, H. Harashima, A. Matsuda
RNA interference (RNAi) induced by siRNAs modified with 4’-thioribonucleosides in cultured mammalian cells.
FEBS Lett. 2005, 579, 3115–3118.
16. Y. Kato, N. Minakawa, Y. Komatsu, H. Kamiya, N. Ogawa, H. Harashima, A. Matsuda
New NTP Analogs: The Synthesis of 4’-ThioUTP and 4’-ThioCTP and Their Utility for SELEX.
Nucleic Acids Res. 2005, 33, 2942–2951.
17. N. Inoue, D. Kaga, N. Minakawa, A. Matsuda
Practical Synthesis of 2’-Deoxy-4’-thioribonucleosides: Substrates for the Synthesis of 4’-thioDNA.
J. Org. Chem. 2005, 70, 8597–8600.
18. S. Hoshika, N. Minakawa, A. Matsuda
Synthesis and physical and physiological properties of 4’-thioRNA: application to post-modification of RNA aptamer toward NF-B.
Nucleic Acids Res. 2004, 32, 3815–3825.
19. N. Minakawa, Y. Kato, K. Uetake, D. Kaga, A. Matsuda
An Improved large scale synthesis of 1,4-anhydro-4-thio-D-ribitol.
Tetrahedron, 2003, 59, 1699–1702.
20. A. B. Smith, III, N. Kanoh, H. Ishiyama, N. Minakawa, J. D. Rainier, R. A. Hartz, Y. S. Cho, H. Cui, W. H. Moser
Tremorgenic Indole Alkaloids. The Total Synthesis of (–)-Penitrem D.
J. Am. Chem. Soc., 2003, 125, 8228–8237.
21. N. Minakawa, N. Kojima, S. Hikishima, T. Sasaki, A. Kiyosue, N. Atsumi, Y. Ueno, A. Matsuda
New Base Paring Motifs. The Synthesis and Thermal Stability of Oligodeoxynucleotides Containing Imidazopyridopyrimidine Nucleosides with the Ability to Form Four Hydrogen Bonds.
J. Am. Chem. Soc., 2003, 125, 9970–9982.
22. N. Minakawa, Y. Ono, A. Matsuda
A Versatile Modification of On-Column Oligodeoxynucleotides Using a Copper-Catalyzed Oxidative Acetylenic Coupling Reaction.
J. Am. Chem. Soc., 2003, 125, 11545–11552.
23. M. Tanimoto, H. Kamiya, N. Minakawa, A. Matsuda, H. Harashima
No Enhancement of Nuclear Entry by Direct Conjugation of a Nuclear Localization signal Peptide to Linearized DNA.
Bioconjugate Chem., 2003, 14, 1197–1202.
24. S. Shuto, N. Minakawa, S. Niizuma, H-S. Kim, Y. Wataya, A. Matsuda
Alternative Synthesis and Antimalarial Effect of (6'R)-6'-C-Methylneplanocin A, a Potent AdoHcy Hydrolase Inhibitor.
J. Med. Chem., 2002, 45, 748–751.
25. J. K. Soukup, N. Minakawa, A. Matsuda, S. A. Strobel
Identification of A-Minor Tertiary Interactions within a Bacterial Group I Intron Active Site by 3-Deazaadenosine Interference Mapping.
Biochemistry, 2002, 41, 10426–10438.
26. N. Minakawa, D. Kaga, Y. Kato, K. Endo, M. Tanaka, T. Sasaki, A. Matsuda
Synthesis and structural elucidation of 1-(3-C-ethynyl-4-thio--D-ribofuranosyl)cytosine (4'-thioECyd).
J. Chem. Soc., Perkin Trans. 1, 2002, 2182–2189.
27. T. Umino, K. Yoshioka, Y. Saitoh, N. Minakawa, H. Nakata, A. Matsuda
Nucleosides and Nucleotides. 200. Reinvestigation of 5’-N-Ethylcarboxamideadenosine Derivatives: Structure-Activity Relationships for P3 purinoceptor-like protein.
J. Med. Chem., 2001, 44, 208–214.
28. K. Shinozuka, R. Ishii-Nozawa, K. Takeuchi, N. Minakawa, A. Matsuda, H. Nakata, M. Kunitomo
Effect of 9-(6,7-Dideoxy--D-allo-hept-5-ynofuranosyl)adenine on Noradrenaline Release from Vascular Sympathetic Nerves.
Clin. Exp. Pharmacol. Physiol., 2001, 28, 312–314.
29. M. Fukuoka, S. Shuto, N. Minakawa, Y. Ueno, A. Matsuda
Synthesis and Biological Activities of Cyclic ADP-carbocyclic-ribose and Its Analogs.
Nucleosides, Nucleotides, Nucleic Acids, 2001, 20, 1355–1358.
30. P. R. Wyde, D. K. Moore-Poveda, E. DeClercq, J. Neyts, A. Matsuda, N. Minakawa, E. Guzman, B. E. Gilbert
Use of Cotton Rats to Evaluate the Efficacy of Antivirals in Treatment of Measles Virus Infections.
Antimicrob. Agents Chemother., 2000, 44, 1146–1152.
31. T. Umino, N. Minakawa, A. Matsuda
Alternative Method to Synthesize 9-(6,7-Dideoxy--D-allo-hept-5-ynofuranosyl)adenine, A Selective and Potent Ligand for P3 Purinoceptor-like Protein: A Stereoselective Reduction Based on Sugar Puckering of the Furanose Ring.
Tetrahedron Lett., 2000, 41, 6419–6423.
32. T. Naka, N. Minakawa, H. Abe, D. Kaga, A. Matsuda
The Stereoselective Synthesis of 4’--Thioribonucleosides via the Pummerer Reaction.
J. Am. Chem. Soc., 2000, 122, 7233–7243.
33. M. Fukuoka, S. Shuto, N. Minakawa, Y. Ueno, A. Matsuda
An Efficient Synthesis of Cyclic IDP- and Cyclic 8-Bromo-IDP-Carbocyclic-Riboses Using a Modified Hata Condensation Method To Form an Intramolecular Pyrophosphate Linkage as a Key Step. An Entry to a General Method for the Chemical Synthesis of Cyclic ADP-Ribose Analogues.
J. Org. Chem., 2000, 65, 5238–5248.
34. N. Kojima, N. Minakawa, A. Matsuda
Studies in the Chemical Conversion of the 4-Carboxamide Group of 5-Amino-1--D-ribofuranosylimidazole-4-carboxamide (AICA-Riboside). Application for the Synthesis of 1-Deazaguanosine.
Tetrahedron, 2000, 56, 7909–7914.
35. M. Fukuoka, S. Shuto, N. Minakawa, Y. Ueno, A. Matsuda
Alternative Synthesis of Cyclic IDP-Carbocyclic Ribose. Efficient Cyclization of an 8-Bromo-N1-[5-(phosphoryl)carbocyclic-ribosyl]inosine 5’-Phenylthiophosphate Derivative Mediated by Iodine.
Tetrahedron Lett., 1999, 40, 5361–5364.
36. T. Naka, N. Nishizono, N. Minakawa, A. Matsuda
Investigation of the Stereoselective Coupling of Thymine with meso-Thiolane-3,4-diol-1-oxide Derivative via the Pummerer Reaction.
Tetrahedron Lett., 1999, 40, 6297–6300.
37. N. Minakawa, N. Kojima, A. Matsuda
Synthesis and Conformational Investigation of Anti-fixed 3-Deaza-3-halopurine Ribonucleosides.
J. Org. Chem., 1999, 64, 7158–7172.
38. A. B. Smith, III, N. Kanoh, N. Minakawa, J. D. Rainier, F. R. Blase, R. A. Hartz
Tremorgenic Indole Alkaloids. Studies Directed Towards the Assembly of the A, F and I Rings of Penitrem D: Observation of an Unexpected Stereochemical Outcome.
Org. Lett., 1999, 1, 1263–1266.
39. T. Mizutani, H. Inagaki, D. Hayasaka, S. Shuto, N. Minakawa, A. Matsuda, H. kariwa, I. Takashima
Transcriptional control of borna disease virus (BDV) in persistently BDV-infected cells.
Arch. Virol., 1999, 144, 1937–1946.
40. N. Minakawa, T. Sasaki, A. Matsuda
Ring-expanded Purine Nucleosides. The synthesis and Cytotoxicity of Imidazo[4,5-c]azepine Nucleosides.
Tetrahedron, 1998, 54, 13517–13528.
41. S. Shuto, M. Kanazaki, S. Ichikawa, N. Minakawa, A. Matsuda
Stereo- and Regioselective Introduction of 1- or 2-Hydroxyethyl Group via Intramolecular Radical Cyclization Reaction with a Novel Silicon-Containing Tether. An Efficient Synthesis of 4’-Branched 2’-Deoxyadenosines.
J. Org. Chem., 1998, 63, 746–754.
42. A. Matsuda, H. Kosaki, Y. Saitoh, Y. Yoshimura, N. Minakawa, H. Nakata
Nucleosides and Nucleotides. 177. 9-(6,7-Dideoxy--D-allo-hept-5-ynofuranosyl)adenine: A Selective and Potent Ligand for P3 Purinoceptor-like Protein.
J. Med. Chem., 1998, 41, 2676–2678.
43. S. Ichikawa, S. Shuto, N. Minakawa, A. Matsuda
Synthesis of 3’--Branched Uridine Derivatives via Intramolecular Reformatsky-type Reaction Promoted by Samarium Diiodide.
J. Org. Chem., 1997, 62, 1368–1375.
44. N. Minakawa, N. Kojima, A. Matsuda
Synthesis of 3-Halo-3-deazainosines: Conformational Lock with the Halogen at the 3-Position of the 3-Deazainosine in Anti-conformation.
Heterocycles, 1996, 42, 149–154.
45. N. Minakawa, N. Kojima, T. Sasaki, A. Matsuda
Synthesis and Antileukemic Activity of 5-Carbon-substituted 1--D-Ribofuranosylimidazole-4-carboxamides.
Nucleosides Nucleotides, 1996, 15, 251–263.
46. N. Minakawa, Y. Sasabuchi, A. Kiyosue, N. Kojima, A. Matsuda
Synthesis of 5-Amino-4-imidazolecarboxamide (AICA) Deoxyribosides from Deoxyinosines and Their Conversion into 3-Deazapurine Derivatives.
Chem. Pharm. Bull., 1996, 44, 288–295.
47. N. Minakawa, A. Matsuda, Z. Xia, L. I. Wiebe, E. E. Knaus
Synthesis of [2-2H]-5-Ethynyl-1-(-D-ribofuranosyl)imidazole-4-carboxamide and 5-Ethynyl-1-([5-3H]--D-ribofuranosyl)imidazole-4-carboxamide (EICAR).
J. Labelled Comp. Radiopharm., 1996, 38, 809–824.
48. W. Wang, V. V. Papov, N. Minakawa, A. Matsuda, K. Biemann, L. Hedstrom
Inactivation of Inosine 5’-Monophosphate Dehydrogenase by the Antiviral Agent 5-Ethynyl-1--D-ribofuranosylimidazole-4-carboxamide 5’-Monophosphate.Biochemistry, 1996, 35, 95–101.
49. A. E. A. Hassan, N. Nishizono, N. Minakawa, S. Shuto, A. Matsuda
Conversion of (Z)-2’-(Cyanomethylene)-2’-deoxyuridines into their (E)-Isomers via Addition of Thiophenol to the Cyanomethylene Moiety Followed by Oxidative Syn-elimination Reactions.
J. Org. Chem., 1996, 61, 6261–6267.
50. A. Ono, N. Haginoya, M. Kiyokawa, N. Minakawa, A. Matsuda
A Novel and Convenient Post-synthetic Modification Method for the Synthesis of Oligodeoxyribonucleotides of 2’-Deoxyuridine.
Bioorg. Med. Chem. Lett., 1994, 4, 361–366.
51. M. Aoyagi, N. Minakawa, A. Matsuda
The synthesis of Imidazo[4,5-e][1,4]diazepine Nucleosides from N1-Substituted Inosines.
Nucleosides Nucleotides, 1994, 13, 1535–1549.
52. Y. Yamagata, M. Kato, M. Aoyagi, N. Minakawa, A. Matsuda
Conformation of 3-Substituted Purine Nucleoside Studied by X-Ray Crystallography and Theoretical Calculations.
Nucleosides Nucleotides, 1994, 13, 1327–1335.
53. N. Minakawa, A. Matsuda
A Convenient Method for the Synthesis of 3-Deazapurine Nucleosides from AICA-riboside.
Tetrahedron Lett., 1993, 34, 661–664.
54. M. Aoyagi, N. Minakawa, A. Matsuda
Synthesis of 3-Alkyl-3-deazainosines via Palladium-catalyzed Intramolecular Cyclization: A New Conformational Lock with the Alkyl Group at the 3-Position of the 3-Deazainosine in Anti-conformation.
Tetrahedron Lett., 1993, 34, 103–106.
55. N. Minakawa, A. Matsuda
Convenient Synthesis of 3-Deazaadenosine, 3-Deazaguanosine, and 3-Deazainosine via Ring Closure of 5-Ethynyl-1--D-ribofuranosylimidazole-4-carboxamide or –carbonitrile.
Tetrahedron, 1993, 49, 557–570.
56. N. Minakawa, T. Sasaki, A. Matsuda
A Transition-state Analogue in Purine Nucleotide Biosynthesis: The Design and Synthesis of an Imidazo[4,5-c]azepine Nucleoside.
Bioorg. Med. Chem. Lett., 1993, 3, 183–186.
57. A. Azuma, Y. Nakajima, N. Nishizono, N. Minakawa, A. Suzuki, K. Hanaoka, T. Kobayashi, M. Tanaka, T. Sasaki, A. Matsuda
2’-C-Cyano-2’-deoxy-1--D-arabinofuranosylcytosine and its Derivatives. A New Class of Nucleoside with a Broad Antitumor Spectrum.
J. Med. Chem., 1993, 36, 4183–4189.
58. A. Matsuda, A. Dan, N. Minakawa, S. J. Tregear, S. Okazaki, Y. Sugimoto, T. Sasaki
Synthesis of 1-(2-Deoxy-2-isocyano--D-arabinofuranosyl)cytosine and Related Nucleosides as Potential Antitumor Agents.
J. Med. Chem., 1993, 36, 4190–4194.
59. N. Minakawa, T. Takeda, T. Sasaki, A. Matsuda, T. Ueda
Synthesis and Antitumor Activity of 5-Ethynyl-1--D-ribofuranosylimidazole-4-carboxamide (EICAR) and Its Derivatives.
J. Med. Chem., 1991, 34, 778–786.
60. E. De Clercq, M. Cools, J. Balzarini, R. Snoeck, G. Andrei, M. Hosoya, S. Shigeta, T. Ueda, N. Minakawa, A. Matsuda
Antiviral Activities of 5-Ethynyl-1--D-ribofuranosylimidazole-4-carboxamide and Related Compounds.
Antimicrob. Agents Chemother., 1991, 35, 679–684.
61. N. Minakawa, A. Matsuda, T. Ueda, T, Sasaki
Synthesis and Antitumor Activies of 5-Ethynylimidazole-4-carboxamide and –carbonitrile Derivatives.
Nucleosides Nucleotides, 1990, 9. 1067–1078.
62. A. Matsuda, N. Minakawa, T. Sasaki, T. Ueda
The Design, Synthesis and Antileukemic Activity of 5-Alkynyl-1--D-ribofuranosylimidazole-4-carboxamide.
Chem. Pharm. Bull., 1988, 36, 2730–2733.
総説・著書
1. 南川 典昭,松田 彰
スーパー核酸:4’-チオ核酸の合成から核酸医薬開発に向けた基礎研究の展開
『化学フロンティア19 創薬をめざす有機合成戦略』(化学同人)2007年,pp157–164.
2. 松田 彰,南川 典昭
核酸医薬の創製をめざして
『バイオとナノの融合 II;新生命科学の応用』(北海道大学COE研究成果編集委員会編;北海道大学)2007年,pp187–198.
3. N. Minakawa, S. Hoshika, N. Inoue, Y. Kato, A. Matsuda
4’-Thionucleic Acids; Chemistry, properties and Applications for Developing Functional Oligonucleotides.
Frontiers in Organic Chemistry: Atta-Ur-Rahman, Y. Hayakawa, Eds.; Bentham Science Publishers LTD., 2005, Vol. 1, p79–102.
4. 南川 典昭
RNAi研究において有機化学ができること:その現状と展望
『RNAi法とアンチセンス法;RNAの科学と応用』(関根光雄・多比良和誠編;講談社サイエンティフィック)2005年、pp110–122.
5. S. Shuto, M. Kanazaki, I. Sugimoto, S. Ichikawa, Y. Nagasawa, Y. Ueno, H. Abe, N. Minakawa, M. Sukeda, T. Kodama, M. Nomura, A. Matsuda
Development of New Radical Reactions with a Vinylsilyl Group and Their Application to the Synthesis of Branched-chain Sugar Nucleosides.
Recent Advances in Nucleosides: Chemistry and Chemotherapy: C. K. Chu, Eds.; Elsevier Science B. V., 2002; p21–55.
6. N. Minakawa, A. Matsuda
Mechanism-based Design of Inosine 5’-Monophosphate Dehydrogenase Inhibitors: Synthesis and Biological Activities of 5-Ethynyl-1--D-ribofuranosylimidazole-4-carboxamide (EICAR) and its Derivatives.
Curr. Med. Chem., 1999, 6, 615–628.
7. 南川 典昭 、松田 彰
イノシン5’–一リン酸脱水素酵素阻害剤と生物活性
蛋白質 核酸 酵素、1995年7月増刊号、40巻、p1299–1305
8. A. Matsuda, A. Azuma, Y. Nakajima, K. Takenuki, A. Dan, T. Iino, Y. Yoshimura, N. Minakawa, T, Sasaki
Design of New Types of Antitumor Nucleosides: The Synthesis and Antitumor Activity of 2’-Deoxy-(2’-C-substituted)cytidines.
Nucleosides and Nucleotides as Antitumor and Antiviral Agents: C. K. Chu, D. C. Baker, Eds.; Plenum Publishing Co.: New York, 1993; p1–22.

